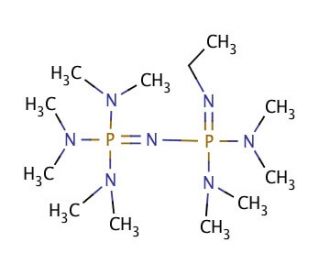
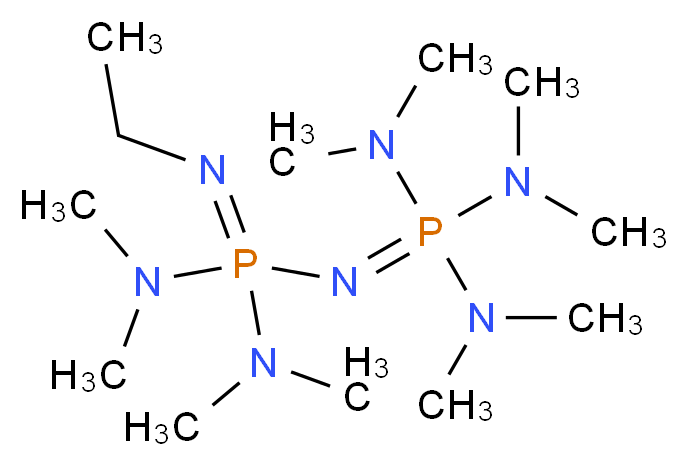
P2Et Phosphazene: A Mild, Functional Group Tolerant Base for Soluble, Room Temperature Pd-Catalyzed C–N, C–O, and C–C Cross-Coupling Reactions | Organic Letters

P2Et Phosphazene: A Mild, Functional Group Tolerant Base for Soluble, Room Temperature Pd-Catalyzed C–N, C–O, and C–C Cross-Coupling Reactions | Organic Letters

P2Et Phosphazene: A Mild, Functional Group Tolerant Base for Soluble, Room Temperature Pd-Catalyzed C-N, C-O, and C-C Cross-Coupling Reactions. | Semantic Scholar
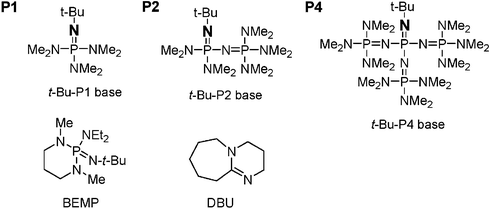
Phosphazene base-catalyzed condensation of trimethylsilylacetate with carbonyl compounds - Chemical Communications (RSC Publishing) DOI:10.1039/B606056K

Tris(2,4,6-trimethoxyphenyl)phosphine - a Lewis base able to compete with phosphazene bases in catalysing oxa-Michael reactions | Organic Chemistry | ChemRxiv | Cambridge Open Engage

P2Et Phosphazene: A Mild, Functional Group Tolerant Base for Soluble, Room Temperature Pd-Catalyzed C–N, C–O, and C–C Cross-Coupling Reactions | Organic Letters

Bifunctional phosphazene-thiourea/urea catalyzed ring-opening polymerization of cyclic esters - ScienceDirect

Organocatalytic Stereoselective Ring-Opening Polymerization of Lactide with Dimeric Phosphazene Bases | Journal of the American Chemical Society
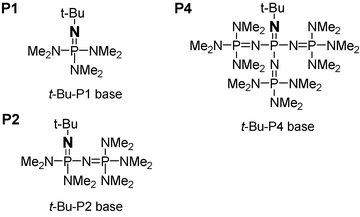
Phosphazene base-promoted halogen –zinc exchange reaction of aryl iodides using diethylzinc - Chemical Communications (RSC Publishing) DOI:10.1039/B605807H
![A Successful Application of Phosphazene Base P2‐tBu to [11C]ABP688 Radiosynthesis in Fully Automated Synthesis Module - Lee - 2020 - Bulletin of the Korean Chemical Society - Wiley Online Library A Successful Application of Phosphazene Base P2‐tBu to [11C]ABP688 Radiosynthesis in Fully Automated Synthesis Module - Lee - 2020 - Bulletin of the Korean Chemical Society - Wiley Online Library](https://onlinelibrary.wiley.com/cms/asset/8d307dde-94b3-4266-9dd3-2fbd42ebce38/bkcs12070-fig-0002-m.jpg)
A Successful Application of Phosphazene Base P2‐tBu to [11C]ABP688 Radiosynthesis in Fully Automated Synthesis Module - Lee - 2020 - Bulletin of the Korean Chemical Society - Wiley Online Library